Mark Staples
2015-2024 - Director, Forest Service Utah Avalanche Center
This article was originally published on Powder.com
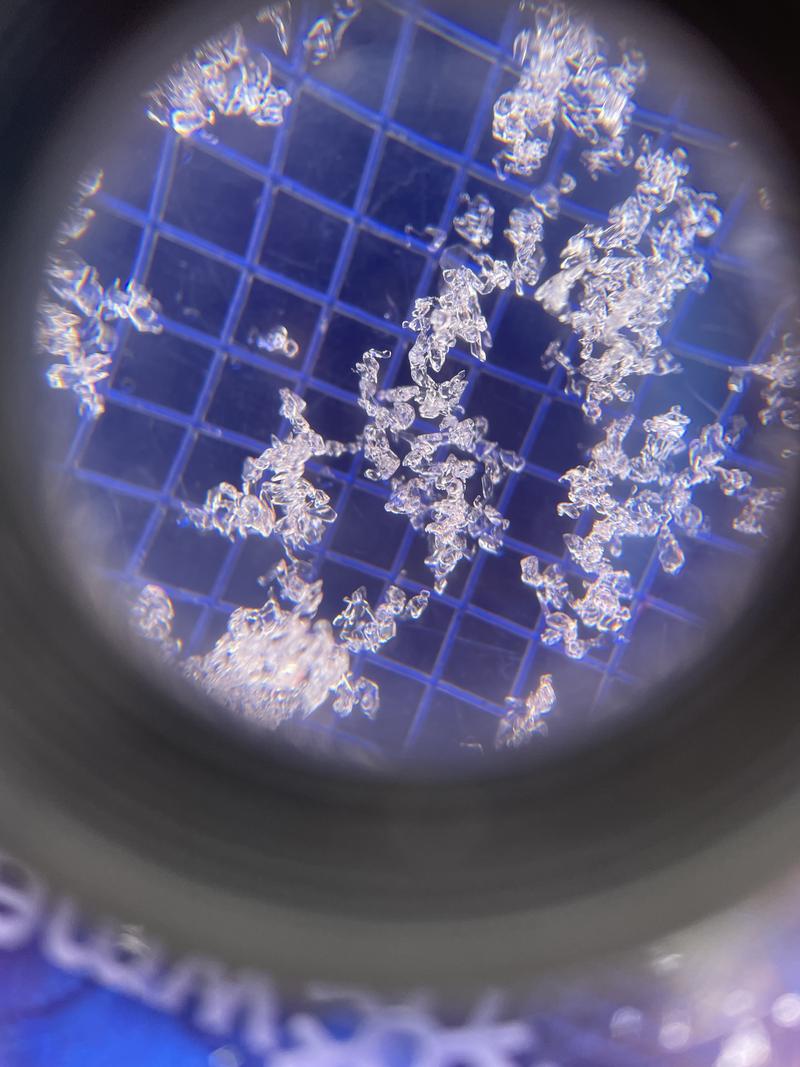

Every little grain of snow changes dramatically, sometimes in a matter of hours, as a result of subtle changes in temperature and pressure.
As skiers and riders, we all know snow. It blankets the mountains with deep powder during the cold winter months offering endless ground for play. But what if I told you that snow is actually a “warm” material? It’s not just warm, it’s hot! Sounds crazy, but understanding this concept can help unlock the science of snow. It can help us understand why powder conditions or avalanche danger can change in the span of days or even just minutes.
Do you remember learning how ice skates work, maybe in high school science class? Essentially ice skates concentrate all our weight on a very narrow blade, putting a lot of pressure on the ice. The ice remains cold, but extra pressure from the blade melts a very thin layer, allowing skaters to effortlessly glide across the ice rink on a thin rail of water.
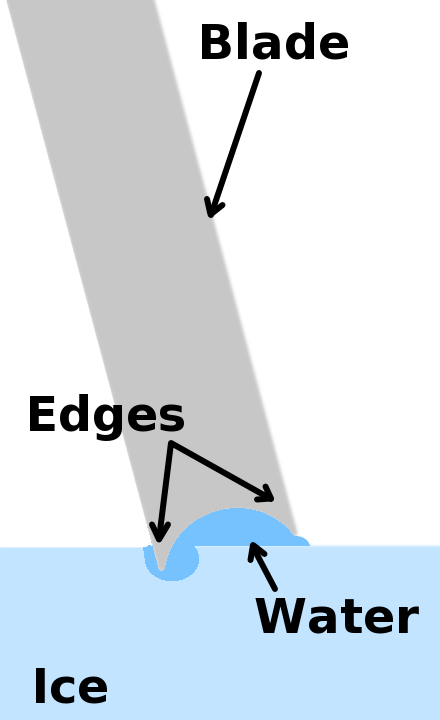
Diagram of an ice skate blade the thin layer of water under it caused by pressure from the weight of the skater. By William R. Wilson - CC BY-SA 3.0, File:Ice Skate Blade Cross Section.png)
Water, like all other matter, exists in three phases - solid, liquid, or vapor. Changing the temperature of water is the easiest way to change between phases. Add heat, and it becomes vapor or steam. Remove the heat, and it becomes a solid or ice. Another way to change the phase is with pressure. Adding a little pressure can melt ice just as ice skates do. Removing pressure can cause water to boil and become steam.
This all means that BOTH temperature and pressure control whether water is a solid, liquid, or gas. If we put those variables onto a graph, we get something called a pressure-temperature diagram or phase diagram. Notice two things in the phase diagram for water. The first is that there is a triple point where water can be either a solid, liquid, or gas at the same time. The second is that the triple point for water occurs very close to common atmospheric temperature and pressure compared to many other materials.
This all means that BOTH temperature and pressure control whether water is a solid, liquid, or gas. If we put those variables onto a graph, we get something called a pressure-temperature diagram or phase diagram. Notice two things in the phase diagram for water. The first is that there is a triple point where water can be either a solid, liquid, or gas at the same time. The second is that the triple point for water occurs very close to common atmospheric temperature and pressure compared to many other materials.
Think about something like steel. At what temperature does steel melt and become a liquid? Over 2000 degrees F! At what temperature does ice or snow melt? 32 degrees. Normal air temperatures in the mountains bounce on either side of that melting temperature all year long.
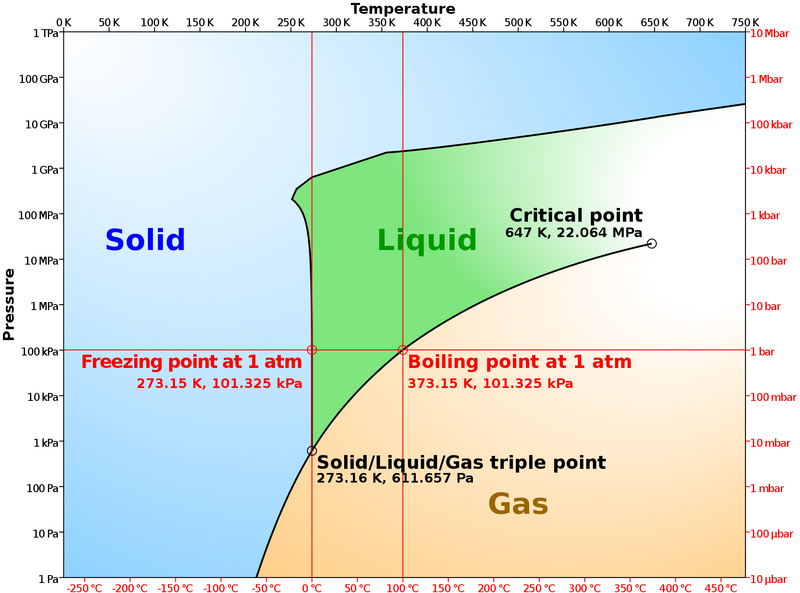 https://www.chemistrylearner.com/phase-diagram-of-water.html
https://www.chemistrylearner.com/phase-diagram-of-water.htmlBecause snow is made of ice which exists near its triple point and its melting point, this entire discussion brings us to one simple concept: snow is a warm material that is always changing. Even though it feels cold to the touch, it is relatively warm because it exists on the verge of melting or even evaporating. The slightest changes in temperature or pressure quickly change it into either a solid, liquid, or vapor, and the shapes of ice crystals in snow is constantly changing.
Ed Adams, retired professor at Montana State University and snow scientist, once said “identifying the properties of snow is like throwing darts blind folded at a moving dart board.” These changes control the type of snow crystals that fall from the sky and determine what kind of powder day we’ll have. These changes also create different snow crystals on the ground, which can create deadly avalanche conditions. Snowmelt and spring runoff are affected by these changes in snow, which can affect how much water we have in reservoirs and whether we have catastrophic wildfires or not. Even the global energy balance is affected by how much solar radiation snow reflects back into the sky. Snow may feel cold, but remember, it’s actually hot!

Forecaster Nikki Champion examining layers and crystals in snow. Understanding how they change helps to forecast avalanche danger.